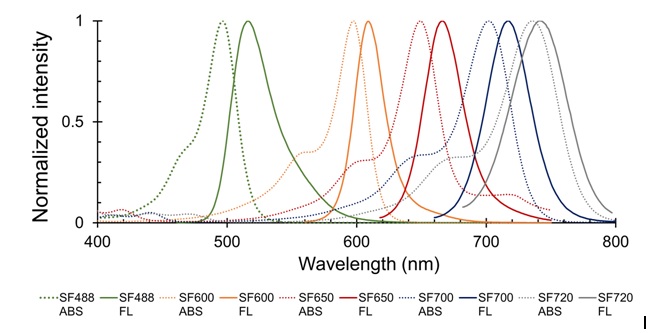
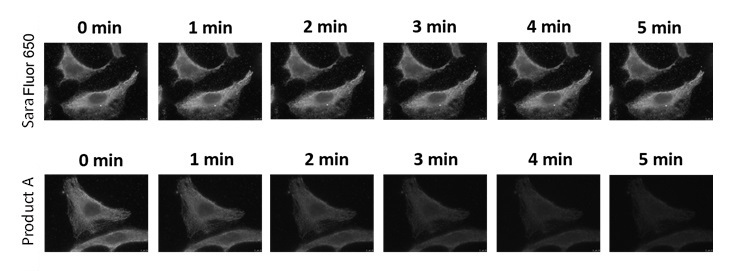
Images using SaraFluor series
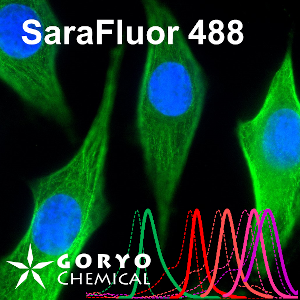
An image using SaraFluor 488-NHS
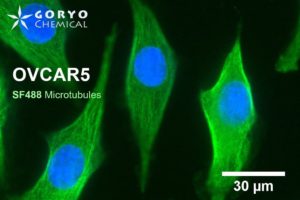
OVCAR5 cells fixed and permeabilized with 1% Triton-X100 were reacted with rabbit anti-α/β tubulin antibody (#2148, Cell Signaling Technology 1/50) and then, reacted with goat anti-rabbit antibody labeled using SaraFluor 488-NHS(0.4 μg/mL, degree of labeling 1.8). Nucleus were stained with Hoechist 33342. The cells were imaged using a fluorescence microscope. Green indicates fluorescence of SaraFluor 488, blue indicates fluorescence of Hoechist 33342.
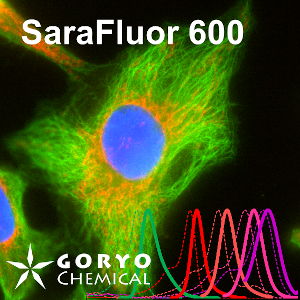
A multicolor imaging example using SaraFluor 600-NHS and 700-NHS
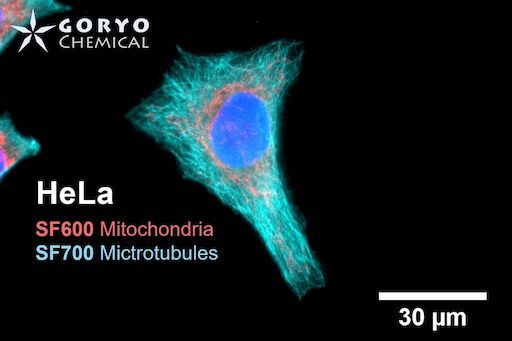
HeLa cells fixed and permeabilized with 1% Triton-X100 were reacted with rabbit anti-COX IV antibody (3E11, Cell Signaling Technology 1/500) and then, reacted with goat anti-rabbit antibody labeled with SaraFluor 600-NHS. In addition, cells were reacted with rat anti-tubulin antibody (YL 1/2, Novus Biologicals, 1/1000) and then, reacted with goat anti-rat antibody labeled with SaraFluor 700-NHS. Nucleus were stained with Hoechist 33342. The cells were imaged using a fluorescence microscope. Red indicates fluorescence of SaraFluor 600, Cyan indicated that of SaraFluor 700, blue indicates fluorescence of Hoechist 33342.
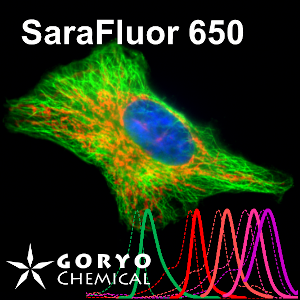
A multicolor imaging example of SaraFluor 488-NHS and 650-NHS
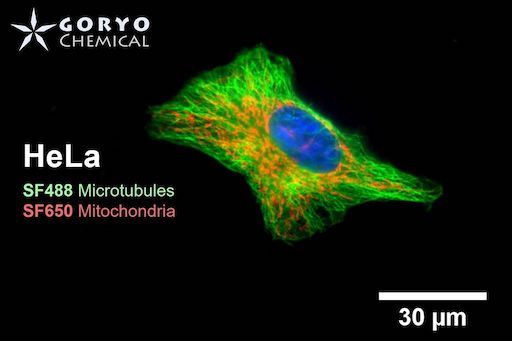
OVCAR5 cells fixed and permeabilized with 1% Triton-X100 were reacted with rabbit anti-α/β tubulin antibody (#2148, Cell Signaling Technology 1/50) and then, reacted with goat anti-rabbit antibody labeled using SaraFluor 488-NHS(0.4 μg/mL, degree of labeling 1.8). Nucleus were stained with Hoechist 33342. The cells were imaged using a fluorescence microscope. Green indicates fluorescence of SaraFluor 488, blue indicates fluorescence of Hoechist 33342.
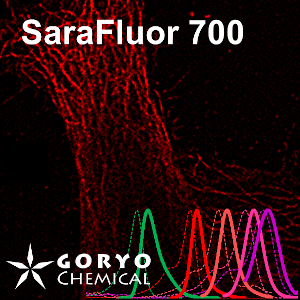
A multicolor imaging example of SaraFluor 600-NHS and 700-NHS

HeLa cells fixed and permeabilized with 1% Triton-X100 were reacted with rabbit anti-COX IV antibody (3E11, Cell Signaling Technology 1/500) and then, reacted with goat anti-rabbit antibody labeled with SaraFluor 600-NHS. In addition, cells were reacted with rat anti-tubulin antibody (YL 1/2, Novus Biologicals, 1/1000) and then, reacted with goat anti-rat antibody labeled with SaraFluor 700-NHS. Nucleus were stained with Hoechist 33342. The cells were imaged using a fluorescence microscope. Red indicates fluorescence of SaraFluor 600, Cyan indicated that of SaraFluor 700, blue indicates fluorescence of Hoechist 33342.
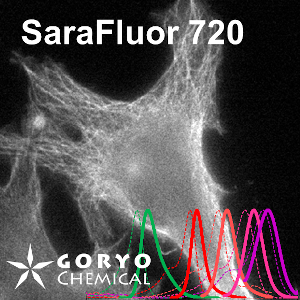
An imaging example of SaraFluor 720-NHS
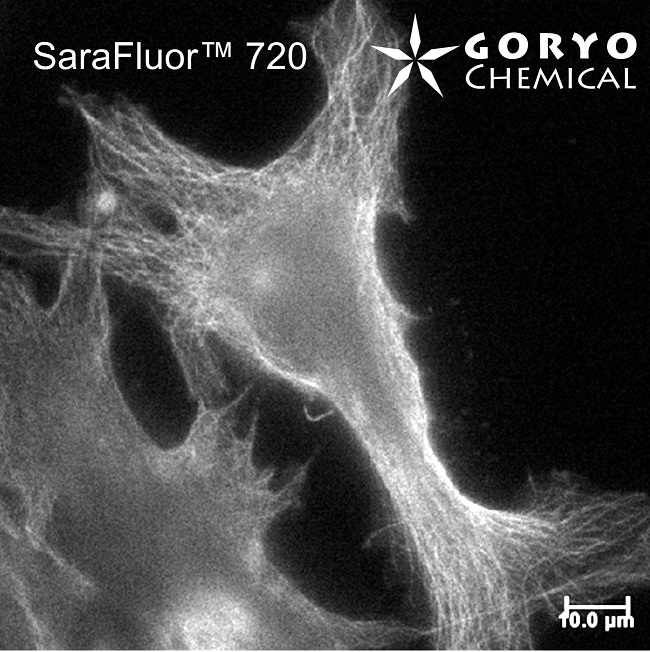
HeLa cells fixed and permeabilized with 1% Triton-X100 were reacted with rat anti-tubulin antibody (YL 1/2, Novus Biologicals, 1/1000) and then, reacted with goat anti-rat antibody labeled using SaraFluor 720-NHS. The cells were imaged using a fluorescence microscope.










 Contact Us
Contact Us
