G. Pezzotti, E. Marin, T. Adachi, A. Rondinella, F. Boschetto, W. Zhu, N. Sugano, R. M. Bock, B. McEntire, S. B. Bal (2017)
Sci. Rep. 7: 44848 DOI:10.1038/srep44848
M. Matsuoka, A. Kumar, M. Muddassar, A. Matsuyama, M. Yoshida, K. Y. J. Zhang (2017)
J. Chem. Inf. Model. 57: 203–213 DOI:10.1021/acs.jcim.6b00649
E. W. Miller, C. J. Chang (2007)
Curr. Opin. Chem. Biol. 11: 620-625 DOI:10.1016/j.cbpa.2007.09.018
N. S. Bryan, M. B. Grisham (2007)
Free Radic. Biol. Med. 43: 645-657 DOI:10.1016/j.freeradbiomed.2007.04.026
A. Doctor, R. Platt, M. L. Sheram, A. Eischeid, T. McMahon, T. Maxey, J. Doherty, M. Axelrod, J. Kline, M. Gurka, A. Gow, B. Gaston (2005)
Proc. Natl. Acad. Sci. U. S. A. 102: 5709-5714 DOI:10.1073/pnas.0407490102
M. M. Tarpey, D. A. Wink, M. B. Grisham (2003)
Am. J. Physiol. Regul. Integr. Comp. Physiol. 286: R431-R444 DOI:10.1152/ajpregu.00361.2003
C. L. Speyer, T. A. Neff, R. L. Warner, R. F. Guo, J. V. Sarma, N. C. Riedemann, M. E. Murphy, H. S. Murphy, P. A. Ward (2003)
Am. J. Pathol. 163: 2319-2328 DOI:10.1016/S0002-9440(10)63588-2
G. Lebuffe, P. T. Schumacker, Z. H. Shao, T. Anderson, H. Iwase, T. L. V. Hoek (2003)
Am. J. Physiol. Heart. Circ. Physiol. 284: H299-H308 DOI:10.1152/ajpheart.00706.2002
X. Zhang, W. S. Kim, N. Hatcher, K. Potgieter, L. L. Moroz, R. Gillette, J. V. Sweedler (2002)
J. Biol. Chem. 277: 48472-48478 DOI:10.1074/jbc.M209130200
M. G. Espey, D. D. Thomas, K. M. Miranda, D. A. Wink (2002)
Proc. Natl. Acad. Sci. U. S. A. 99: 11127-11132 DOI:10.1073/pnas.152157599
N. Weiss, Y. Y. Zhang, S. Heydrick, C. Bierl, J. Loscalzo (2001)
Proc. Natl. Acad. Sci. U. S. A. 98: 12503-12508 DOI:10.1073/pnas.231428998
M. G. Espey, K. M. Miranda, D. D. Thomas, D. A. Wink (2001)
J. Biol. Chem. 276: 30085-30091 DOI:10.1074/jbc.M101723200
R. C. Kuo, G. T. Baxter, S. H. Thompson, S. A. Stricker, C. Patton, J. Bonaventura, D. Epel (2000)
Nature. 406: 633-636 DOI:10.1038/35020577
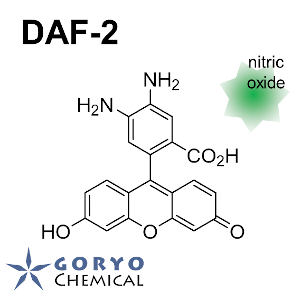
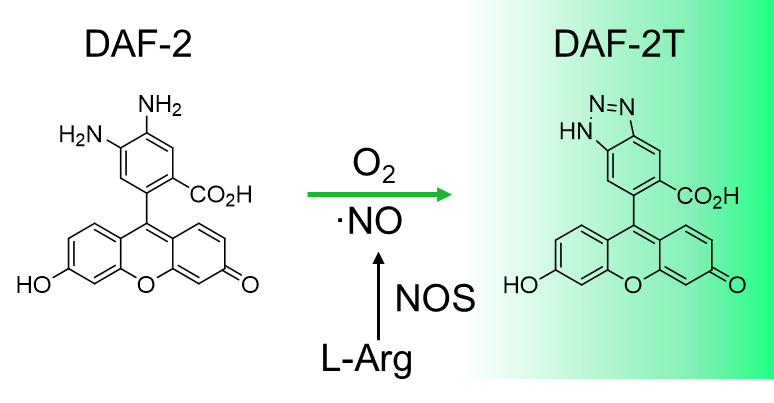
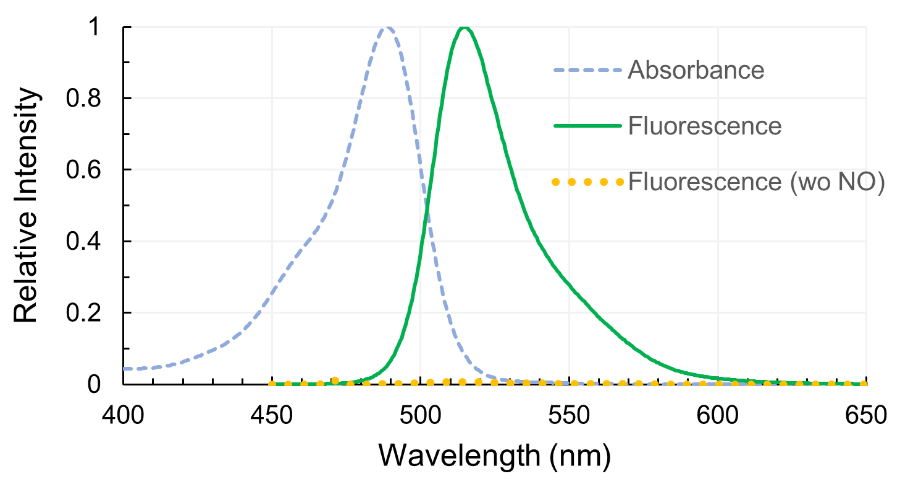
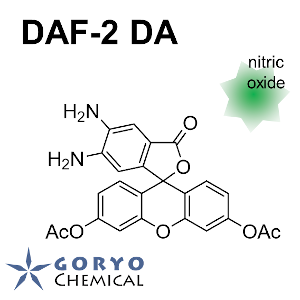
N. Nakatsubo, H. Kojima, K. Kikuchi, H. Nagoshi, Y. Hirata, D. Maeda, Y. Imai, T. Irimura, T. Nagano (1998)
FEBS Lett. 427: 263-266 DOI:10.1016/S0014-5793(98)00440-2
H. Kojima, N. Nakatsubo, K. Kikuchi, S. Kawahara, Y. Kirino, H. Nagoshi, Y. Hirata, T. Nagano (1998)
Anal. Chem. 70: 2446-2453 DOI:10.1021/ac9801723







 Contact Us
Contact Us
