T. Karasawa, A. Kawashima, F. Usui-Kawanishi, S. Watanabe, H. Kimura, R. Kamata, K. Shirasuna, Y. Koyama, A. Sato-Tomita, T. Matsuzaka, H. Tomoda, S. Y. Park, N. Shibayama, H. Shimano, T. Kasahara, M. Takahashi (2018)
Arterioscler. Thromb. Vasc. Biol. 38: 744–756 DOI:10.1161/ATVBAHA.117.310581
S. Kitazawa, S. Nishizawa, H. Nakagawa, M. Funata, K. Nishimura, T. Soga, T. Hara (2017)
Cancer Sci. 108: 1185-1193 DOI:10.1111/cas.13240
K. Kitakaze, Y. Mizutani, E. Sugiyama, C. Tasaki, D. Tsuji, N. Maita, T. Hirokawa, D. Asanuma, M. Kamiya, K. Sato, M. Setou, Y. Urano, T. Togawa, A. Otaka, H. Sakuraba, K. Itoh (2016)
J. Clin. Invest. 126: 1691-1703 DOI:10.1172/JCI85300
A. Hayashi, D. Asanuma, M. Kamiya, Y. Urano, S. Okabe (2016)
Neuropharmacology 100: 66-75 DOI:10.1016/j.neuropharm.2015.07.026
D. Asanuma, Y. Takaoka, S. Namiki, K. Takikawa, M. Kamiya, T. Nagano, Y. Urano, K. Hirose (2014)
Angew. Chem. Int. Ed. Engl. 53: 6085-6089 DOI:10.1002/anie.201402030
M. Isa, D. Asanuma, S. Namiki, K. Kumagai, H. Kojima, T. Okabe, T. Nagano, K. Hirose (2014)
ACS. Chem. Biol. 9: 2237-2241 DOI:10.1021/cb500654q
R. Watanabe, N. Soga, D. Fujita, K. V. Tabata, L. Yamauchi, S. H. Kim, D. Asanuma, M. Kamiya, Y. Urano, H. Suga, H. Noji (2014)
Nat. Commun. 5: 4519 DOI:10.1038/ncomms5519
![]()


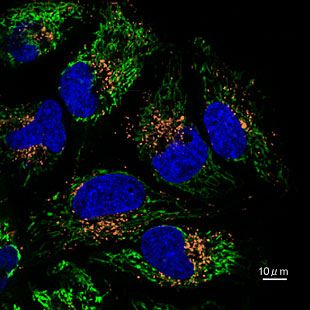




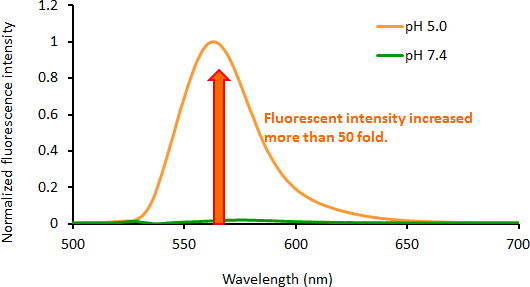
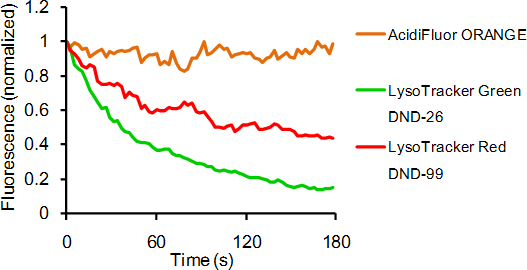
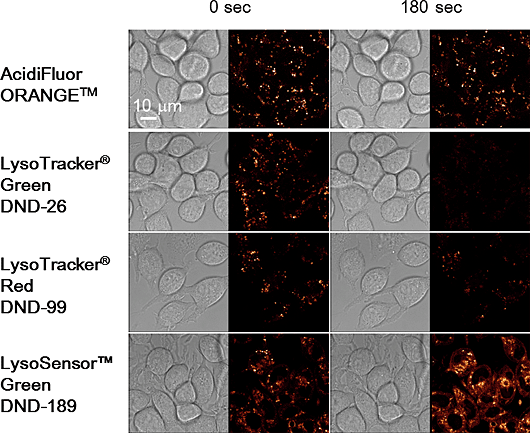
 Contact Us
Contact Us
